By John Ikani
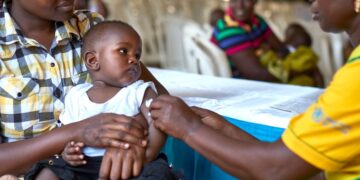
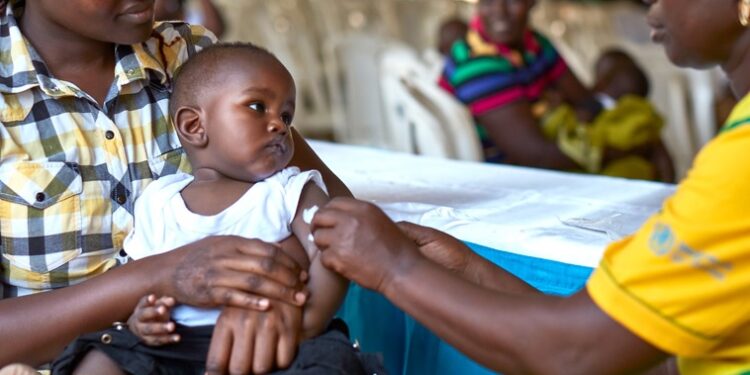
The World Health Organization (WHO) has given its endorsement to a new malaria vaccine, potentially offering a more affordable and accessible alternative to the first malaria shot.
The R21/Matrix-M vaccine, developed by Oxford University in the UK, has received WHO’s approval to combat the life-threatening disease transmitted by certain mosquitoes.
WHO Director-General Tedros Adhanom Ghebreyesus, in a moment of enthusiasm, expressed his joy, stating, “As a malaria researcher, I used to dream of the day we would have a safe and effective vaccine against malaria. Now we have two.”
The R21/Matrix-M vaccine, produced by the Serum Institute of India, has already secured approval for use in Burkina Faso, Ghana, and Nigeria.
It will be introduced in these African nations in early 2024, with availability in other regions by mid-2024. The cost of each dose is expected to range from $2 to $4, making it an affordable option for many.
Tedros also revealed that WHO is currently assessing the vaccine for prequalification, which would enable organizations like GAVI and UNICEF to purchase it directly from manufacturers, ensuring wider distribution.
Oxford University, in collaboration with the Serum Institute of India, developed this new three-dose vaccine. Research indicates it is over 75 percent effective, with protection lasting for at least a year when a booster shot is administered.
While these malaria vaccines are promising, it’s crucial to note that neither of them stops transmission. Therefore, immunization campaigns alone may not suffice to halt malaria epidemics.
In addition, efforts to combat the disease face challenges due to increasing drug resistance and the spread of invasive mosquito species.
In a related development, the WHO recommended Takeda Pharmaceuticals’ dengue vaccine for children aged six to 16 living in areas where dengue is a significant public health concern.
Dengue, a viral infection transmitted by mosquitoes, is prevalent in tropical and subtropical regions.
Takeda’s vaccine, known as Qdenga, has shown effectiveness against all four serotypes of the virus in individuals previously infected with dengue.
However, its performance against serotypes 3 and 4 in those without prior infections remains uncertain.
Bangladesh has been grappling with a severe dengue outbreak, with nearly 1,000 deaths reported this year, highlighting the urgency of effective prevention and treatment measures.
Lastly, the WHO’s strategic advisory group recommended a simplified single-dose regimen for most COVID-19 vaccines, aiming to enhance vaccine acceptance as many individuals have already experienced at least one infection.
The streamlined approach seeks to streamline the vaccination process and increase its accessibility to the general population.